Evidence-Based Nutritional Supplementation in Polycystic Ovary Syndrome: A Comprehensive Review of Clinical Outcomes, Mechanisms, and Recommendations
Nusly Andres-Montenegro
1 Facultad de ciencias, (Universidad Nacional Autónoma de Honduras/ Tegucigalpa /
Honduras); nusly.andres@unah.hn .
2 Facultad de ciencias, (Universidad Nacional Autónoma de Honduras/ Tegucigalpa /
Honduras); sidnee.molina@unah.hn
3 Instituto de Investigaciones en Microbiología, (Universidad Nacional Autónoma de Honduras/ Tegucigalpa/Honduras).
* Correspondence: olinda.nunez@unah.edu.hn; Tel.: (+504) 9856-8413
ABSTRACT
Polycystic Ovary Syndrome (PCOS) is a prevalent and multifactorial endocrine-metabolic disorder among women of reproductive age. Recent research indicates that nutritional supplementation may play a valuable adjunctive role in PCOS management, particularly in addressing metabolic, hormonal, and inflammatory disturbances. This review synthesizes evidence from randomized controlled trials and meta-analyses published up to 2024, evaluating the efficacy and safety of commonly used supplements such as myo-inositol, vitamin D, omega-3 fatty acids, selenium, and coenzyme Q10. The analysis demonstrates that several supplements can improve insulin sensitivity, lipid profiles, hormonal balance, and oxidative stress markers in women with PCOS. Mechanistic insights suggest these effects are mediated through the modulation of metabolic and inflammatory pathways, including AMPK, NF-κB, PPAR-γ, and VDR.
Nevertheless, the heterogeneity of study designs, sample sizes, and a paucity of long-term safety data highlight the need for standardized protocols and further high-quality trials. Overall, nutritional supplements offer a promising strategy for personalized PCOS management, especially for women with insulin resistance or those seeking alternatives to conventional pharmacotherapy. Continued research into optimal dosing, duration, and co-supplementation effects will be essential to refine clinical recommendations and maximize patient outcomes. This is the most comprehensive synthesis, combining clinical outcomes with mechanistic insights across 13 key nutritional supplements in PCOS management.
Keywords. Polycystic Ovary Syndrome, Nutritional Supplements, Inositol, Omega-3 Fatty Acids, Coenzyme Q10, Vitamin D, Selenium, Insulin Resistance, Clinical Trials, Evidence-Based Review, Endocrine Metabolism
Keywords. Polycystic Ovary Syndrome, Nutritional Supplements, Inositol, Omega-3 Fatty Acids, Coenzyme Q10, Vitamin D, Selenium, Insulin Resistance, Clinical Trials, Evidence-Based Review, Endocrine Metabolism
INTRODUCTION
Polycystic Ovary Syndrome (PCOS) is a multifactorial endocrine-metabolic disorder that affects approximately 6–20% of women of reproductive age, depending on diagnostic criteria¹. It is characterized by hyperandrogenism, oligo/anovulation, and polycystic ovarian morphology, often accompanied by insulin resistance, dyslipidemia, and low-grade chronic inflammation². Due to its complex etiology and heterogeneous presentation, PCOS requires a multidisciplinary management approach³. While pharmacological treatments remain the standard of care, there is growing interest in adjunctive non-pharmacological strategies, including dietary and lifestyle modifications⁴. Various nutritional supplements have emerged as potential modulators of metabolic, hormonal, and inflammatory pathways involved in PCOS pathophysiology⁵. These supplements are thought to exert their effects through various mechanisms, including improving insulin sensitivity, reducing oxidative stress, and modulation of androgen production and inflammatory cytokine levels⁶. A comprehensive evaluation of these supplements within evidence-based frameworks is essential to guide their safe and effective integration into clinical practice⁷.
Supplements And Polycystic Ovary Syndrome
The treatment for polycystic ovary syndrome (PCOS) has focused on drugs that allow control over androgen production and insulin resistance. However, a comprehensive treatment is required, as it is a condition that can trigger various metabolic complications 5. Traditionally, in PCOS, one of the main aspects to consider is hyperglycemia, as it can trigger type 2 diabetes mellitus, cardiovascular diseases, the production of inflam-matory factors, and reactive oxygen species that cause oxidative stress and tissue damage 5; Moreover, hy-perandrogenism and insulin resistance significantly affect lipid metabolism in PCOS 6. So, women with PCOS are prone to developing dyslipidemia 7, which includes elevated concentrations of a variety of serum lipids but reduced levels of HDL cholesterol 8. To date, various supplements have been used as part of the treatment and management of PCOS to achieve a better therapeutic response and reduce complications 5. Next, we describe them:
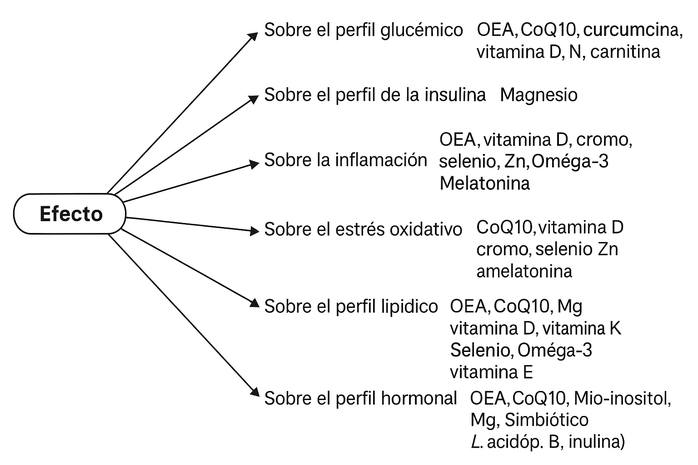
Figure 1. Represents the effect of various supplements on polycystic ovary syndrome. Five impact areas are identified: glycemic profile, inflammation, oxidative stress, lipid profile, and hormonal profile, each linked to metabolic and endocrine alterations characteristic of PCOS. Within each category, supplements with therapeutic potential are listed.
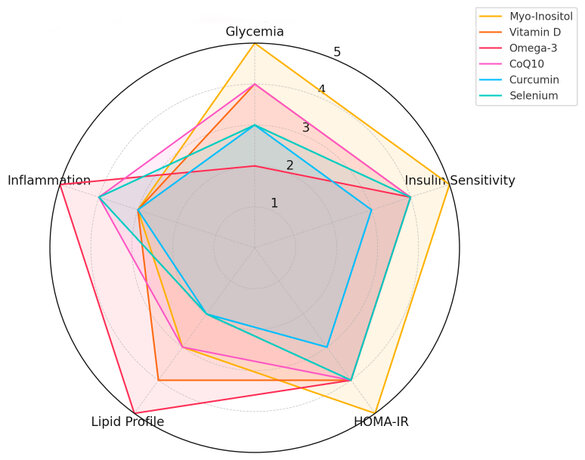
Figure 2. Mechanisms of action of dietary supplements in PCOS treatment.
A simplified schematic representation of the molecular and physiological pathways influenced by the main supplements reviewed. The illustration highlights their interaction with key metabolic and endocrine targets such as AMPK, PPAR-γ, NF-κB, VDR, and oxidative stress markers. These mechanisms improve glucose metabolism, hormonal balance, and inflammation and reduce insulin resistance.
OLEOILETANOLAMIDE
Oleoylethanolamide (OEA) is a lipid derived from oleic acid, possessing anti-inflammatory and antioxidant properties. It can be absorbed from the diet or synthesized by the human body from oleic acid 5. Oral sup-plementation with OEA has been shown to influence the levels of Anti-Müllerian Hormone (AMH), fasting blood sugar (FBS), the Homeostasis Model Assessment for Insulin Resistance (HOMA IR), tumor necrosis factor-alpha (TNF-α), and C-reactive protein (CRP), significantly decreasing their serum concentration starting from a dose of 125 mg/day, over eight weeks 5. The effect on blood glucose is associated with in-creased insulin sensitivity 5. Regarding the levels of CRP and TNF-α, different studies suggest that their decrease is due to the suppression of the NF-κB pathway (nuclear factor kappa-light-chain-enhancer of ac-tivated B cells) and the activation of signals through PPAR-α (peroxisome proliferator-activated receptor alpha). Regarding the effects on oxidation, it has been shown to decrease malondialdehyde (MDA) and, in turn, increase total antioxidant capacity (TAC) levels 5. According to various authors, this reduction is sig-nificant when administering 125 to 300 mg of OEA 5 doses.
COENZYME Q10
Coenzyme Q10 (CoQ10) is a liposoluble benzoquinone that supplements by scavenging free radicals, inhib-iting protein oxidation, and regulating lipid metabolism markers and glycemic control in patients with type 2 diabetes mellitus 9 . A study conducted by Samimi et al. 9 demonstrated that a dose of 100 mg/day of CoQ10 to patients with PCOS for 12 significantly improved glycemic control, insulin metabolism markers, total cholesterol, and LDL. CoQ10 acts under a blocking effect on interleukins such as IL-1B, inhibiting insulin secretion stimulated by glucose. Its intake contributes to improving insulin resistance through the modulation of insulin and adiponectin receptors and the enhancement of glucose transporters 10 . Regarding the lipid profile, the consumption of CoQ10 could lead to an improvement by reducing oxidative stress and its im-plication in endothelial metabolism 9.
CURCUMIN
Curcumin (diferuloylmethane) is an active polyphenolic pigment from Curcuma longa, and it possesses an-tioxidant and anti-inflammatory properties 11. Due to its pharmacological and biological characteristics, its hypoglycemic and hypolipidemic effects made it a supplement of interest 12. Different mechanisms are pro-posed to explain curcumin's effect on glucose homeostasis, which are mainly based on the activation of the gamma receptor, which is activated by the peroxisome proliferator (PPAR-y). This exerts control over genes involved in metabolic homeostasis and simultaneously stimulates the action of enzymes such as glucokinase 12. According to the study by Heshmati et al. 11, curcumin increases glucose absorption by activating AMP-activated protein kinase, thereby increasing phosphorylation. The intake of capsules containing 500 mg of curcumin powder (1500 mg per day) for 12 weeks significantly decreases glucose and DHEA levels in patients with PCOS11. In the study by Sohaei et al.12 1 g of curcumin (500 mg twice a day) was administered daily for 6 weeks, resulting in a significant decrease in serum insulin levels and QUICK, although no statis-tical significance was observed in other glycemic parameters.
VITAMIN D
Vitamin D is a steroid hormone whose function is regulated by the vitamin D receptor (VDR), which is found in various tissues of the human body, including the ovaries, playing an important role in female reproductive physiology 13. It has been evidenced that vitamin D improves glucose metabolism, as it increases insulin production and receptor expression reduces the production of pro-inflammatory cytokines 7. Various studies, including a bibliographic review, indicate that the most favorable results were achieved with high doses of vitamin D (≥4000 IU/d) over a minimum period of 12 weeks. This dosage improved glucose levels, insulin sensitivity, hyperlipidemia, and hormonal function 14. Insulin resistance correlates in most cases where there is a vitamin D deficiency, as it has been shown to be capable of modulating the homeostasis between glucose and insulin due to the action it exerts on the VDR. These receptors activate the transcription of the human insulin receptor gene, thereby stimulating the expression of insulin receptors improving insulin-mediated glucose transport 14. Significant changes have been recorded in glucose levels, serum insulin, HOMA-IR, CRP, and MDA following supplementation with doses of 50,000 IU of vitamin D every two weeks for 12 weeks 15. A meta-analysis demonstrated that vitamin D supplementation in women with PCOS affects hs-CRP, MDA, and TAC but did not affect NO or GSH 16 levels.
Vitamin D may have a reducing effect on lipid metabolism parameters 6. Studies suggest an association between low serum vitamin D concentrations and an unfavorable lipid profile in patients with PCOS8. However, supplementation has a greater hypolipidemic effect in patients with metabolic disorders and varies by population6. A study showed that vitamin D supplemented for 12 weeks in women with PCOS yielded significant results in reducing insulin concentration and HOMA-IR at fasting, 1 hour, 2 hours, and 3 hours after the OGTT7. The lipid profile showed a significant reduction in triglycerides, total cholesterol, and lipoprotein compared to the control group 7. The exact mechanism by which vitamin D improves lipid markers is still under debate6. It is attributed to vitamin D stimulating the expression of the LPL gene in muscles and adipose tissue, increasing the clearance of circulating lipoprotein particles 6. Wen et al.7 indicated that vitamin D can increase hepatic intracellular calcium, which stimulates microsomal triglyceride transfer protein (MTP), which is involved in the formation and secretion of VLDL, resulting in a decrease in serum levels of triglycerides and VLDL cholesterol. Other studies associate the effect of vitamin D with the reduction of parathyroid hormone and the improvement of insulin sensitivity 6, suggesting that vitamin D intake could reduce cholesterol absorption, thereby affecting the serum concentration of Total Cholesterol and the endogenous synthesis of cells 6.
VITAMIN K
It is a fat-soluble vitamin synthesized naturally, phylloquinone (vitamin K1) from green vegetables and menaquinone (vitamin K2) produced by intestinal bacteria 17
MELATONIN
Melatonin is a hormone secreted by the pineal gland; it performs various functions, controlling sleep patterns, adjusting the circadian rhythm, contributing to the immune response, protecting oocytes from ROS, and is also considered a free radical eliminator and an endogenous antioxidant 19 . It regulates oxidative damage and attenuates the inflammatory state through anti-inflammatory enzymes and eliminating free radicals in the tissues 19 . To date, no significant changes have been observed in glucose, insulin, cholesterol, triglycerides, LDL, or HDL; however, there are heterogeneous results regarding the changes that may occur in PCR and MDA levels 19 . The doses of melatonin that have been used range between 3 and 10 mg/day for 3 to 12 weeks 19 .
INOSITOL
Inositols belong to the B vitamin complex; there are nine stereoisomers, the most important being Myo-inositol and D-chiro-inositol. These are considered an alternative treatment to metformin, as their function lies in the modulation of the components that signal the insulin pathways, thus influencing processes such as the regulation of the menstrual cycle, carbohydrate metabolism, and hyperandrogenism 20 . Myo-inositol in the human body promotes the translocation of glucose transporter type 4 to the plasma membrane for glucose uptake and, in turn, reduces the release of free fatty acids 21 . Additionally, it produces messengers of inositol triphosphates, which regulate the production of hormones such as TSH and FSH and are also responsible for glucose absorption, thereby benefiting insulin sensitivity 22 22 . Regarding the D-chiro-inositol isoform, it has a positive effect on hyperandrogenism indices, but it does not significantly reduce glucose levels 23 . However, both isoforms have demonstrated their effectiveness in ovarian function and metabolism in patients with PCOS 23 .
CHROMIUM
Chromium is a trace element that benefits the endocrine profile, inflammation biomarkers, and oxidative stress 24 . These effects are attributed to the action of enzymes, such as the activation of glutathione reductase, the improvement of insulin resistance, and the inhibition of protein glycosylation 24 . It has been demonstrated that the intake of 200 μg/day of chromium picolinate for 8 weeks significantly reduces hirsutism, CRP-as, MDA, and TAC; however, it did not influence the plasma concentrations of NO and GSH 24 . The exact mechanism by which chromium influences CRP levels is not precisely known, but it is suggested that its inhibitory effect on pro-inflammatory cytokines is related to the reduction of oxidative stress, which decreases CRP24 . Chromium has an insulinotropic impact inhibiting epinephrine, which could be related to the decrease in oxidative stress biomarkers 24 . The intake of chromium supplements has been shown to improve insulin metabolism in PCOS patients, decreasing serum insulin levels, HOMA IR, HOMA B, and significantly increasing QUICKI, but it does not affect glucose levels 25 .
SELENIUM
It is an essential trace element with antioxidant properties, fundamental for selenoproteins that regulate the function of endothelial cells and protect against oxidative damage 26 . A randomized, double-blind, placebo-controlled trial conducted by Razavi et al. 27 found that supplementation with 200 μg of selenium for 8 weeks significantly increased pregnancy rates and reduced levels of alopecia, acne, DHEA, hirsutism, CRP, and MDA compared to placebo. It is said that antioxidants can influence female reproduction by eliminating or suppressing the formation of ROS, free radicals, and lipid hydroperoxides 27 . The effect of the decrease in serum levels of dehydroepiandrosterone (DHEA) and hirsutism occurs by improving insulin metabolism markers 27 . Similarly, selenium supplementation reduces serum levels of CRP by inhibiting the activation of NF-kappa B through the modulation of selenoprotein gene expression and the increase in its biosynthesis, resulting in a suppressed production of CRP 27 . A study conducted by Jamilian et al.28 reported that 200 μg of selenium for 8 weeks of intervention in women with PCOS had significantly reduced levels of insulin, HOMA-IR, HOMA-B, triglycerides, VLDL, and a significant increase in QUICK compared to placebo. Also, Jamilian et al. suggest that the improvement in insulin parameters could be due to the inhibition of COX-2 expression, P-selectin, or inflammatory cytokines such as TNF-α and IL-1. Other studies have shown that selenium stimulates the expression of the pancreatic beta cell gene and improves islet function in cell cultures, suggesting its antidiabetic potential due to its insulin-like properties 28 .
ZINC
It is an essential trace element in prominent sources of both animal and plant origin 29 . Zinc (Zn) is required to maintain homeostasis, serving as a catalytic, structural, or regulatory ion for approximately 3000 proteins in the body 29 . It has antioxidant properties and protective actions against ROS in collaboration with other antioxidants, such as vitamin E 29 . A study conducted by Jamilian et al.30 demonstrated that the administration of 50 mg/day of zinc in patients with PCOS for 8 weeks significantly reduced hirsutism, alopecia, MDA, and CRP; however, it did not affect hormonal profiles, inflammatory cytokines, or other oxidation biomarkers. The intervention with 220 mg of zinc sulfate (containing 50 mg of zinc) for 8 weeks reduced fasting serum glucose and insulin levels and improved HOMA-IR, HOMA-B, and QUICK31 .
Additionally, a decrease in the concentration of triglycerides and VLDL cholesterol was observed in subjects with PCOS 31 . Zinc is an insulin mimetic as it stimulates lipogenesis and glucose uptake in isolated adipocytes; this effect occurs through the modulation of protein tyrosine phosphatase activity in IGF-1 signaling 17 . It performs a regulatory action on insulin phosphorylation and its impact on oxidative stress 29 . Regarding triglyceride and VLDL cholesterol levels, it has been suggested that Zn affects the enzymes involved in lipid metabolism 31 .
L-CARNITINE
Carnitine (LC) is a quaternary amine synthesized endogenously from lysine and methionine. Among its multiple functions, LC is a mandatory cofactor in transporting long-chain fatty acids across the mitochondrial membrane, facilitating beta-oxidation 32 . LC can regulate androgen levels and, in turn, contribute to the decrease of testosterone. Just as it improves insulin sensitivity, thus affecting androgen levels and ovarian hormones 32 . A study reports that a daily dose of 250 mg of carnitine supplement for 12 weeks among women with PCOS resulted in a significant reduction in body weight, BMI, fasting glucose, serum insulin, HOMA-IR, and DHEA but did not affect lipid profiles or free testosterone 33 . The improvement in insulin metabolism parameters is associated with L-carnitine potentially modulating the expression of glycolytic and gluconeogenic enzymes involved in mitochondrial glucose oxidation and facilitating the transport of free fatty acids or their action as acetyl group donors in high-energy metabolism situations 33 .
OMEGA-3
Omega-3 fatty acids are polyunsaturated fatty acids (PUFAs) in dietary supplements such as fish oil. The three main omega-3 fatty acids are alpha-linolenic acid, eicosapentaenoic acid (EPA), and docosahexaenoic acid (DHA)17 . They are attributed to anti-inflammatory, antioxidant, and antihypertensive properties and regulate the abnormal expression of some genes in polycystic ovary syndrome 17 . It is under debate whether there is a relationship between PCOS, omega-3 fatty acids, and insulin resistance 34 . Several studies suggest that omega-3 supplementation improves glucose parameters, possibly due to the potential of fatty acids to increase adiponectin production 34 . Adiponectin is a hormone that enhances cell sensitivity to insulin and has anti-inflammatory and anti-atherogenic effects 34 .
Melo et al.34 reported that supplementation with 1000 mg/day of omega-3 from flaxseed oil for 12 weeks showed effective results, even more so than in similar studies. The consumption of omega-3 based on flaxseed oil favored the reduction of insulin, HOMA-IR, CRP, and the increase in QUICKI 35 . There was also a reduction in triglycerides and VLDL cholesterol. However, I do not observe any significant effect on hormonal profiles, other lipid profiles, and nitric oxide levels 35 . Regarding the improvement of insulin sensitivity, omega-3 fatty acids inhibit the activation of nuclear factor kappa B (NF-kB), which reduces the production of pro-inflammatory cytokines and helps reverse insulin resistance 35 . Omega-3 supplementation seems to be an option that can be effective in improving the lipid profile and reducing cardiovascular risk, as metabolic nuclear receptors like PPAR and SREBP-1 (sterol regulatory element-binding protein 1) are activated by the binding of natural lipid-derived ligands such as fatty acids, allowing the inhibition of protein-coding that stimulates lipid synthesis and genes that increase lipid oxidation in the liver and muscle 34 . Moreover, the consumption of omega-3 is associated with activating the AMPK (AMP-activated protein kinase) pathway, which acts as a sensor of the cellular energy state that regulates energy homeostasis between lipid oxidation and lipogenesis 34 . A randomized, parallel-arm, double-blind study was conducted on 51 women with PCOS, showing a significant improvement in the triglyceride lipid profile and the high-density lipoprotein (HDL) ratio34 . The effects of n-3 PUFAs on lipid metabolism may be class-specific, as fish oil and flaxseed oil decrease triglyceride concentration 36 . In 6 months of intervention with a supplemental dose of 2 g/day of omega-3 in women with PCOS, they showed a reduction in total cholesterol, LDL, and triglycerides and a significant increase in HDL cholesterol 34,37 .
MAGNESIUM
It is an intracellular ion and a cofactor for hundreds of enzymes involved in regulating the effects of insulin 17 . Due to its mechanisms of regulating glycemic and neurological functions, magnesium is associated with insulin resistance and depression, including polycystic ovary syndrome, as well as some cardiovascular diseases and diabetes17 . A systematic review found that high magnesium levels were associated with a general decrease in insulin resistance (IR), based on four epidemiological studies in women with polycystic ovary syndrome (PCOS). However, when analyzing the results of three randomized clinical trials (RCTs), the effects of magnesium supplementation on IR in these women were variable. However, when analyzing the results of three randomized clinical trials (RCTs), the impact of magnesium supplementation on IR in these women was variable 17 .
VITAMIN E
It is a fat-soluble nutrient with anticoagulant and antioxidant properties that help protect cells from damage caused by free radicals 17 . A study reported that supplementation with vitamin E alone for 8 weeks significantly decreased serum triglyceride2 . However, there was no effect on HDL or LDL levels 38 . The exact mechanism of action of vitamin E on the lipid profile is unknown 38 . One of the hypotheses attempting to explain this is that alterations in oxidative stress mediate these effects. Consequently, chronic exposure to oxidants conditions cellular metabolism by oxidizing lipids 38 . It is claimed that vitamin E has antioxidant, anti-inflammatory, and anti-obesity properties 38 .
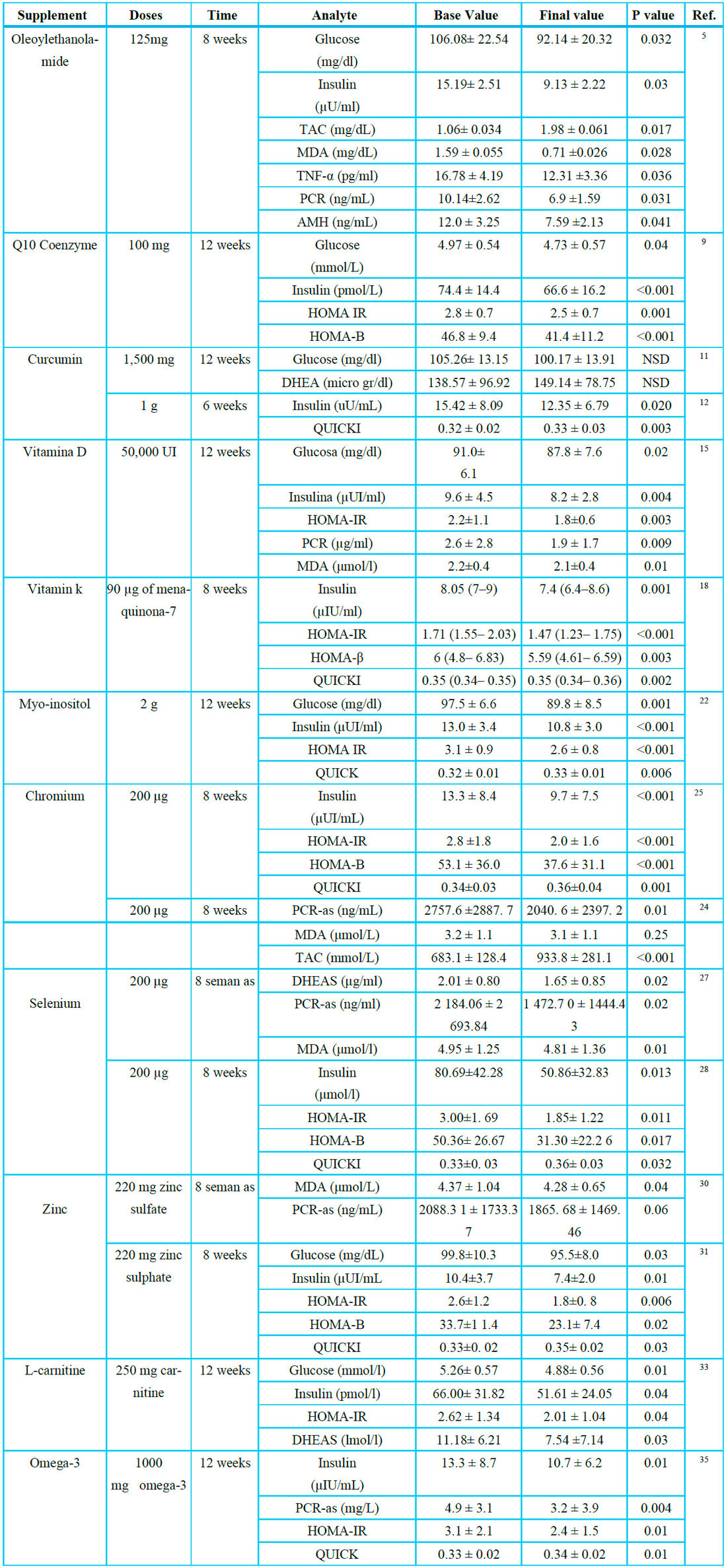
*N/A: Not available
Table 1. Summary of supplements for glycemic control, oxidative stress, and insulin resistance
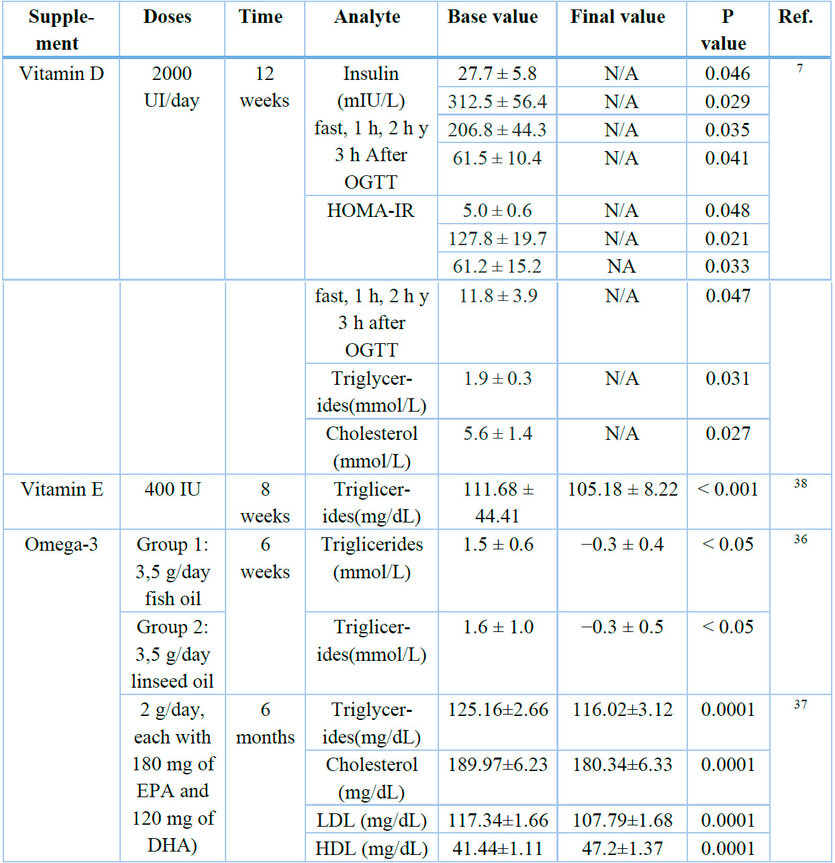
*N/A: Not Available
Table 2. Summary of supplements used in the control of dyslipidemia
Various authors have linked the gut microbiome and PCOS, as a deficient microbiome can influence the progression of this syndrome due to hyperandrogenism, impaired signaling through epithelial receptors, disorders in the gut-brain axis, and increased secretion of inflammatory cytokines 39 . Recently, probiotics and prebiotics have been studied as treatments for dysbiosis 39 .
Symbiotics, a combination of probiotics and prebiotics, develop a stimulating function that is selective for the growth and activation of beneficial intestinal bacteria 39 . It has been shown that administering probiotics, prebiotics, and synbiotics to women with PCOS benefits glycemic status, insulin resistance, and lipid profile 39 . As part of the studies recorded to date, microorganisms such as Bacillus coagulans (GBI-30), Lactobacillus rhamnosus, Lactobacillus acidophilus, Lactobacillus casei, Bifidobacterium bifidum, and fructooligosaccharides have been used, which have managed to improve the quality of life concerning health, total testosterone, SHBG, hs-CRP, TAC, and MDA 39 .
Table 3. Summary of hormonal, inflammatory, and oxidative stress biomarkers following 12-week supplementation with a symbiotic combination (L. acidophilus, L. casei, B. bifidum, and inulin) in women with PCOS. The table presents changes in serum levels of testosterone, SHBG, DHEAS, hs-CRP, nitric oxide (NO), total antioxidant capacity (TAC), glutamine, and malondialdehyde (MDA) before and after treatment. All values are expressed as mean ± standard deviation. N/A: P-values were not reported in the original study. Data adapted from reference 39.

Table 3. Summary of probiotics and symbiotics.
This literature review defined and compared thirteen supplements to treat and manage PCOS as an alternative to traditional drugs, identifying their main effects, the most appropriate doses for each, and the most favorable time periods to achieve significant results. Among the main findings of the research, we highlight that the supplements of greatest clinical relevance in the treatment of PCOS are characterized by being aimed at the progressive improvement of the most frequent clinical manifestations in patients, thus encompassing four important parameters to achieve an efficient result: glycemic control, dyslipidemia control, reduction of oxidative stress, and hormonal control (Figure 1). Although there is significant evidence regarding the contribution of different supplements to these parameters, it seems that there is no "ideal" supplementation that improves all these elements of PCOS at once. However, Omega 3 is the most promising as it demonstrates modulatory effects on the lipid profile (TGC, LDL-C, VLDL-C, and HDL-C) and insulin resistance (HOMA-IR). The limitation of Omega 3 is that it appears to have no significant effect on glycemia. No adverse effects have been reported from using Omega 3, being considered relatively safe, although authors like Melo et al.34 y Xia et al. 40 recommend larger-scale studies with long-term follow-up to evaluate possible adverse effects. However, it is recommended to use this supplementation in women with PCOS who present both insulin resistance and dyslipidemia 41 .
On the other hand, OEA and CoQ10 have a positive effect on glucose control by increasing insulin sensitivity. It has been evidenced that administering 125 mg of OEA or ingesting 100 to 200 mg of CoQ10 for 8 to 12 weeks significantly reduces serum glucose and insulin levels in patients with PCOS 5 . Establishing the dose of Co Q10 has been a topic of discussion; authors identify a greater beneficial effect on total cholesterol when doses below 400 mg/day are used 42 . CoQ10 supplementation has proven to be safe and well-tolerated even at high doses, but rare adverse effects such as nausea, vomiting, loss of appetite, and dyspepsia have been reported 43 .
For its part, Inositol has proven to be one of the supplements with the best effect in treating PCOS, being comparable in most parameters to metformin 20 . Both isoforms, Myo-Inositol and D-chiro Inositol, have shown effectiveness in glycemic metabolism and ovarian function. Myo-inositol shows improvement in glycemic/metabolic profile at the ovarian level by modulating glucose metabolism and FSH signaling, while D-chiro shows improvement in signs of hyperandrogenism 44 . Therefore, a combined dose of Myo-Inositol and D-chiro inositol (40:1) is recommended to improve both aspects of PCOS. Regarding adverse effects, Inositol has proven to be a safe treatment; evidence shows that there may be a higher risk of adverse effects in women using metformin compared to those using inositol 45 .
On the other hand, in our bibliographic research, we have found evidence of a beneficial effect of certain supplements on dyslipidemia or insulin resistance but not directly on PCOS, so these could be the subject of future studies. These are vitamin K, melatonin, chromium, selenium, magnesium, and vitamin E. Regarding vitamin K, we could say that there are few studies on the effect of this vitamin on PCOS, as studies are often designed using co-supplementation with vitamin D and calcium, showing that this co-supplementation improves hyperandrogenism and antioxidant status 46 and has a positive effect on insulin resistance in women with PCOS 47 . On the other hand, the improvement of depressive state in women with PCOS has been reported when supplemented with vitamin K2 48 . Lastly, another supplement with interesting evidence is selenium. As we explained earlier, selenium supplementation improves the state of hyperandrogenism, hirsutism, oxidative status, and insulin resistance in women with PCOS. Also, it seems promising as an antidiabetic treatment for enhancing the function of pancreatic beta cells 49,50 .
Critical Analysis of the Evidence
Despite the valuable information provided by the current review, it is necessary to consider the variability and limitations of the scientific evidence supporting the use of supplements to treat and manage PCOS. One of the major challenges encountered is the heterogeneity in study design, sample size, population characteristics, and dosage and duration of supplementation. For instance, while some trials use standardized doses over controlled periods, others vary considerably in their protocols, making it difficult to compare their outcomes51 directly.
In addition, many studies do not control for confounding variables such as diet, lifestyle, and baseline hormonal levels, which may significantly influence the effectiveness of supplementation52. This issue is particularly relevant in cases of co-supplementation or the simultaneous use of pharmacological treatment, which often obscures the isolated effect of the supplement under investigation 53.
Another important factor is the lack of long-term data. Most studies reviewed are short-term (typically 6 to 12 weeks) and do not provide information on the sustainability of the clinical benefits or potential adverse effects of prolonged use. For example, while selenium and vitamin D have shown promising results in short-term trials, their long-term impact on endocrine and metabolic parameters in PCOS remains unclear54.It is also notable that some supplements show inconsistent results across studies. A clear example is melatonin, which presents mixed findings regarding its impact on glucose and lipid profiles55. These inconsistencies may reflect the differences in study methodologies and the need for more rigorous clinical trials with standardized endpoints and larger sample sizes.
Lastly, while several supplements show strong potential (such as Inositol and omega-3 fatty acids), only a few have sufficient evidence from randomized controlled trials to support their clinical recommendation. This underscores the importance of continuing high-quality research that can clarify which supplements are most beneficial, under what circumstances, and for which subgroups of PCOS patients.
To better understand the reliability of the reviewed studies, we conducted a methodological quality assessment based on key design features, including randomization, blinding, use of control groups, and sample size. The table below summarizes the quality grading for the main trials considered in this review. Studies with complete reporting and rigorous design were rated as "High," while others with some limitations or missing methodological details were rated as "Medium" or "Low."
To evaluate the reliability and scientific rigor of the evidence presented in this review, we conducted a methodological quality assessment of the primary clinical trials included. The evaluation focused on essential design elements such as sample size, randomization procedures, blinding, presence of a control group, and overall risk of bias. The table below summarizes the core methodological features and provides a quality rating for each study, helping to contextualize their contribution to clinical recommendations.

Table 4. A methodological quality assessment of the clinical trials is included in this review. The table presents the characteristics of eleven randomized controlled trials evaluating the efficacy of nutritional supplements in women with PCOS. Quality was graded based on the presence of randomization, blinding, control groups, and completeness of reporting. Studies were classified as High, Medium, or Low quality. Data extracted from sources referenced in the review.
Practical Recommendations for Supplement Use in PCOS
Based on the evidence reviewed, we developed a set of practical recommendations to guide the potential use of dietary supplements in clinical settings. These recommendations consider the main clinical targets in PCOS (insulin resistance, lipid imbalance, hormonal dysfunction), the most effective dosages and durations, and the current level of supporting evidence. The table below is intended to help clinicians make decisions while acknowledging the need for individualized treatment plans.
Based on the reviewed evidence, a practical summary was developed to guide clinicians in selecting dietary supplements tailored to specific clinical presentations of PCOS. This table compiles the most commonly studied supplements, their suggested dosages and durations, clinical focus, and strength of supporting evidence. It is intended as a quick reference tool to aid in formulating personalized and evidence-based treatment strategies.
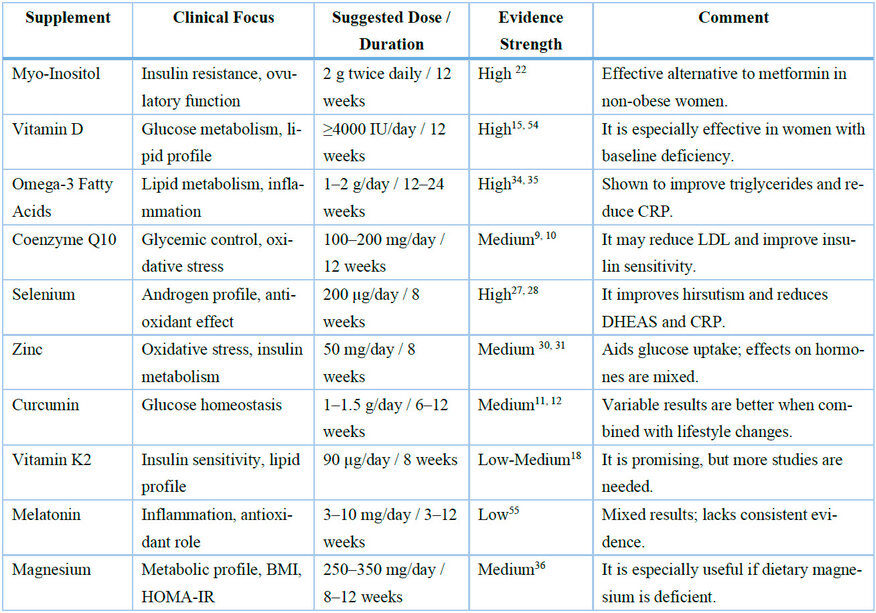
Table 5. Practical recommendations for the clinical use of nutritional supplements in PCOS. This table summarizes key supplements by clinical indication, recommended dosage, duration, and available evidence's strength. The strength of evidence was rated as High, Medium, or Low, based on trial design and reproducibility of results.
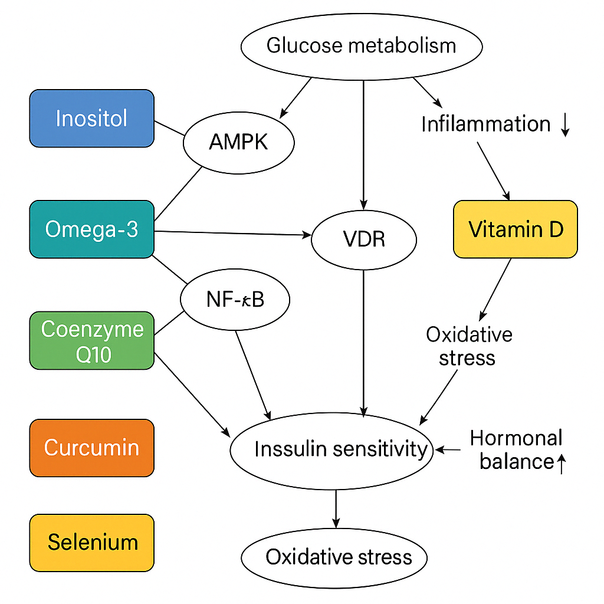
Figure 3. Comparative radar chart of the clinical efficacy of selected supplements in PCOS management. The graph illustrates the relative performance of six key supplements—Myo-Inositol, Vitamin D, Omega-3, Coenzyme Q10, Curcumin, and Selenium—across five critical outcome domains: glycemic control, insulin sensitivity, HOMA-IR, lipid profile improvement, and reduction of inflammation. Data are synthesized from clinical trials and categorized on a scale from 0 (no effect) to 5 (strong effect). This visualization supports quick clinical comparison and decision-making.
Clinical Limitations and Safety Considerations of Supplement Use
While nutritional supplements offer promising benefits for the management of PCOS, several limitations and potential risks must be acknowledged to provide a balanced interpretation of the evidence.
Firstly, the safety profiles of many supplements are not fully established, especially for long-term use. Although short-term trials show minimal adverse effects, the prolonged intake of agents such as selenium, vitamin D, and zinc can lead to toxicity if not monitored adequately58. For instance, high doses of selenium have been associated with selenosis, which manifests with gastrointestinal disturbances, hair loss, and neurological symptoms59. Similarly, hypervitaminosis D may lead to hypercalcemia, nephrocalcinosis, and vascular calcifications60.
Secondly, potential drug-supplement interactions must be considered, especially in women undergoing pharmacological treatments for PCOS or comorbidities. For example, supplements like omega-3 fatty acids and vitamin E have anticoagulant properties that might interact with medications such as oral contraceptives or metformin61.
Another critical aspect is the variability in supplement formulations. Different brands and preparations can vary widely in purity, bioavailability, and actual content compared to what is declared on the label62. This inconsistency may affect clinical outcomes and raise concerns about study results' reproducibility.
Moreover, individual variability among patients can influence supplement efficacy. Factors such as baseline nutritional status, gut microbiome composition, genetic polymorphisms, and severity of PCOS symptoms may alter the response to supplementation63. This emphasizes the necessity of a personalized approach rather than a one-size-fits-all recommendation.
Finally, the lack of regulatory oversight in many countries regarding dietary supplements compared to pharmaceutical drugs increases the risk of contamination or mislabeling64, further highlighting the importance of selecting supplements from reputable manufacturers.
CONCLUSIONS
This literature review highlights the growing interest in dietary supplements as a complementary approach to treating and managing Polycystic Ovary Syndrome (PCOS). By synthesizing data from clinical trials and reviews, we categorized the most clinically relevant supplements according to their primary effects—glycemic control, lipid regulation, oxidative stress reduction, and hormonal balance—while also detailing their recommended doses and treatment durations.
The evidence suggests that Inositol, vitamin D, omega-3 fatty acids, coenzyme Q10, curcumin, and oleoyl ethanolamide benefit insulin sensitivity, glucose metabolism, and inflammatory markers. Other supplements like selenium, zinc, L-carnitine, probiotics, and vitamin K show potential in improving endocrine-metabolic profiles, though more high-quality research is needed to confirm their efficacy and safety.
However, researchers have conducted many studies with small sample sizes, short intervention periods, and frequent use of co-supplementation, which limits the ability to isolate the effects of individual compounds. Most studies fail to include direct statistical comparisons between supplements or report long-term safety outcomes. Moreover, inconsistent study designs further complicate the clinical translation of findings.
Despite these challenges, nutritional supplementation remains promising, particularly for patients seeking personalized or alternative therapies. For optimal results, clinicians should consider individualized approaches, monitor for possible adverse effects, and ensure supplementation is integrated into a broader treatment plan grounded in evidence-based practice.
Future research should focus on:
- Conducting large-scale, randomized controlled trials with rigorous methodological quality.
- Investigating long-term outcomes and safety profiles.
- Clarifying mechanisms of action for each supplement.
- Exploring potential interactions with conventional pharmacotherapy.
By addressing these gaps, the scientific community can better define the role of dietary supplements in PCOS and contribute to improved quality of life and clinical outcomes for women affected by this complex syndrome.
Author Contributions: Conceptualization, Olinda Núñez Murillo.; Methodology, Olinda Núñez Murillo and Nusly Andres Montenegro.; software, Nusly Andres Montenegro and Sidnee Molina Gutierrez; validation, Olinda Núñez Murillo, Nusly Andres Montenegro and Sidnee Molina Gutierrez; formal analysis, Olinda Núñez Murillo.; investigation, Nusly Andres Montenegro and Sidnee Molina Gutierrez; resources, Nusly Andres Montenegro and Sidnee Molina Gutierrez; data curation, Nusly Andres Montenegro and Sidnee Molina Gutierrez; writing—original draft preparation, Nusly Andres Montenegro and Sidnee Molina Gutierrez; writing—review and editing, Olinda Núñez Murillo; visualization, Olinda Núñez Murillo.; supervision, Olinda Núñez Murillo.; project administration, Olinda Núñez Murillo. All authors have read and agreed to the published version of the manuscript.
Funding: This research received no external funding.
Institutional Review Board Statement: Not applicable.
Informed Consent Statement: Not applicable.
Conflicts of Interest: The authors declare no conflict of interest.
REFERENCES
1. Concha CF, Recabarren SE, Pérez BF. Epigenética del síndrome de ovario poliquístico. Rev Med Chil. 2017;145(7):907-15. doi:10.4067/s0034-98872017000700907.
2. Heidari H, Hajhashemy Z, Saneei P. A meta-analysis of effects of vitamin E supplementation alone and in combination with omega-3 or magnesium on polycystic ovary syndrome. Sci Rep. 2022;12:19927. doi:10.1038/s41598-022-24467-0.
3. Ortiz AE, Luque M, Escobar HF. Polycystic ovary syndrome in adult women. Med Clin (Barc). 2019;152(10):450-7. doi:10.1016/j.medcli.2018.11.019.
4. Kiani AK, Donato K, Dhuli K, Stuppia L, Bertelli M. Dietary supplements for polycystic ovary syndrome. J Prev Med Hyg. 2022;63(2 Suppl 3):E206-E213. doi:10.15167/2421-4248/jpmh2022.63.2S3.2762.
5. Shivyari FT, Pakniat H, Nooshabadi MR, Rostami S, Haghighian HK, Shiri-Shahsavari MR. Examining the oleoylethanolamide supplement effects on glycemic status, oxidative stress, inflammation, and anti-müllerian hormone in polycystic ovary syndrome. J Ovarian Res. 2024;17(1):111. doi:10.1186/s13048-024-01432-1.
6. Luo J, Li T, Yuan J. Effectiveness of vitamin D supplementation on lipid profile in polycystic ovary syndrome women: a meta-analysis of randomized controlled trials. Ann Palliat Med. 2021;10(1):114-29. doi:10.21037/apm-20-2492.
7. Wen X, Wang L, Li F, Yu X. Effects of vitamin D supplementation on metabolic parameters in women with polycystic ovary syndrome: a randomized controlled trial. J Ovarian Res. 2024;17(1):147. doi:10.1186/s13048-024-01473-6.
8. Jin B, Qian L, Fu X, Zhu J, Shu J. Influence of vitamin D supplementation on lipid levels in polycystic ovary syndrome patients: a meta-analysis of randomized controlled trials. J Int Med Res. 2020;48(6):300060520935313. doi:10.1177/0300060520935313.
9. Samimi M, Zarezade Mehrizi M, Foroozanfard F, Akbari H, Jamilian M, Ahmadi S, et al. The effects of coenzyme Q10 supplementation on glucose metabolism and lipid profiles in women with polycystic ovary syndrome: a randomized, double-blind, placebo-controlled trial. Clin Endocrinol (Oxf). 2017;86(4):560-6. doi:10.1111/cen.13288.
10. Izadi A, Ebrahimi S, Shirazi S, Taghizadeh S, Parizad M, Farzadi L, et al. Hormonal and Metabolic Effects of Coenzyme Q10 and/or Vitamin E in Patients With Polycystic Ovary Syndrome. J Clin Endocrinol Metab. 2019;104(2):319-27. doi:10.1210/jc.2018-01221.
11. Heshmati J, Moini A, Sepidarkish M, Morvaridzadeh M, Salehi M, Palmowski A, et al. Effects of curcumin supplementation on blood glucose, insulin resistance and androgens in patients with polycystic ovary syndrome: A randomized double-blind placebo-controlled clinical trial. Phytomedicine. 2021;80:153395. doi:10.1016/j.phymed.2020.153395.
12. Sohaei S, Amani R, Tarrahi MJ, Ghasemi-Tehrani H. The effects of curcumin supplementation on glycemic status, lipid profile and hs-CRP levels in overweight/obese women with polycystic ovary syndrome: A randomized, double-blind, placebo-controlled clinical trial. Complement Ther Med. 2019;47:102201. doi:10.1016/j.ctim.2019.102201.
13. Irani M, Merhi Z. Role of vitamin D in ovarian physiology and its implication in reproduction: a systematic review. Fertil Steril. 2014;102(2):460-8.e3. doi:10.1016/j.fertnstert.2014.04.046.
14. Menichini D, Facchinetti F. Effects of vitamin D supplementation in women with polycystic ovary syndrome: a review. Gynecol Endocrinol. 2020;36(1):1-5. doi:10.1080/09513590.2019.1625881.
15. Maktabi M, Chamani M, Asemi Z. The Effects of Vitamin D Supplementation on Metabolic Status of Patients with Polycystic Ovary Syndrome: A Randomized, Double-Blind, Placebo-Controlled Trial. Horm Metab Res. 2017;49(6):493-8. doi:10.1055/s-0043-107242.
16. Akbari M, Ostadmohammadi V, Lankarani KB, Tabrizi R, Kolahdooz F, Heydari ST, et al. The Effects of Vitamin D Supplementation on Biomarkers of Inflammation and Oxidative Stress Among Women with Polycystic Ovary Syndrome: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Horm Metab Res. 2018;50(4):271-9. doi:10.1055/s-0044-101355.
17. Alesi S, Ee C, Moran LJ, Rao V, Mousa A. Nutritional Supplements and Complementary Therapies in Polycystic Ovary Syndrome. Adv Nutr. 2022;13(4):1243-66. doi:10.1093/advances/nmab141.
18. Tarkesh F, Namavar Jahromi B, Hejazi N, Tabatabaee H. Beneficial health effects of Menaquinone-7 on body composition, glycemic indices, lipid profile, and endocrine markers in polycystic ovary syndrome patients. Food Sci Nutr. 2020;8(10):5612-21. doi:10.1002/fsn3.1837.
19. Ziaei S, Hasani M, Malekahmadi M, Daneshzad E, Kadkhodazadeh K, Heshmati J. Effect of melatonin supplementation on cardiometabolic risk factors, oxidative stress and hormonal profile in PCOS patients: a systematic review and meta-analysis of randomized clinical trials. J Ovarian Res. 2024;17(1):138. doi:10.1186/s13048-024-01450-z.
20. Greff D, Juhász AE, Váncsa S, Váradi A, Sipos Z, Szinte J, et al. Inositol is an effective and safe treatment in polycystic ovary syndrome: a systematic review and meta-analysis of randomized controlled trials. Reprod Biol Endocrinol. 2023;21(1):10. doi:10.1186/s12958-023-01055-z.
21. Fitz V, Graca S, Mahalingaiah S, Liu J, Lai L, Butt A, et al. Inositol for Polycystic Ovary Syndrome: A Systematic Review and Meta-analysis to Inform the 2023 Update of the International Evidence-based PCOS Guidelines. J Clin Endocrinol Metab. 2024;109(6):1630-55. doi:10.1210/clinem/dgad762.
22. Shokrpour M, Foroozanfard F, Afshar Ebrahimi F, Vahedpoor Z, Aghadavod E, Ghaderi A, et al. Comparison of myo-inositol and metformin on glycemic control, lipid profiles, and gene expression related to insulin and lipid metabolism in women with polycystic ovary syndrome: a randomized controlled clinical trial. Gynecol Endocrinol. 2019;35(5):406-11. doi:10.1080/09513590.2018.1540570.
23. Genazzani AD, Santagni S, Rattighieri E, Chierchia E, Despini G, Marini G, et al. Modulatory role of D-chiro-inositol (DCI) on LH and insulin secretion in obese PCOS patients. Gynecol Endocrinol. 2014;30(6):438-43. doi:10.3109/09513590.2014.897321.
24. Jamilian M, Bahmani F, Siavashani MA, Mazloomi M, Asemi Z, Esmaillzadeh A. The Effects of Chromium Supplementation on Endocrine Profiles, Biomarkers of Inflammation, and Oxidative Stress in Women with Polycystic Ovary Syndrome: a Randomized, Double-Blind, Placebo-Controlled Trial. Biol Trace Elem Res. 2016;172(1):72-8. doi:10.1007/s12011-015-0570-6.
25. Jamilian M, Asemi Z. Chromium Supplementation and the Effects on Metabolic Status in Women with Polycystic Ovary Syndrome: A Randomized, Double-Blind, Placebo-Controlled Trial. Ann Nutr Metab. 2015;67(1):42-8. doi:10.1159/000438465.
26. Rashidi BH, Mohammad Hosseinzadeh F, Alipoor E, Asghari S, Yekaninejad MS, Hosseinzadeh-Attar MJ. Effects of Selenium Supplementation on Asymmetric Dimethylarginine and Cardiometabolic Risk Factors in Patients with Polycystic Ovary Syndrome. Biol Trace Elem Res. 2020;196(2):430-7. doi:10.1007/s12011-019-01954-6.
27. Razavi M, Jamilian M, Kashan ZF, Heidar Z, Mohseni M, Ghandi Y, et al. Selenium Supplementation and the Effects on Reproductive Outcomes, Biomarkers of Inflammation, and Oxidative Stress in Women with Polycystic Ovary Syndrome. Horm Metab Res. 2016;48(3):185-90. doi:10.1055/s-0035-1559604.
28. Jamilian M, Razavi M, Fakhrie Kashan Z, Ghandi Y, Bagherian T, Asemi Z. Metabolic response to selenium supplementation in women with polycystic ovary syndrome: a randomized, double-blind, placebo-controlled trial. Clin Endocrinol (Oxf). 2015;82(6):885-91. doi:10.1111/cen.12699.
29. Nasiadek M, Stragierowicz J, Klimczak M, Kilanowicz A. The Role of Zinc in Selected Female Reproductive System Disorders. Nutrients. 2020;12(8):2464. doi:10.3390/nu12082464.
30. Jamilian M, Foroozanfard F, Bahmani F, Talaee R, Monavari M, Asemi Z. Effects of Zinc Supplementation on Endocrine Outcomes in Women with Polycystic Ovary Syndrome: a Randomized, Double-Blind, Placebo-Controlled Trial. Biol Trace Elem Res. 2016;170(2):271-8. doi:10.1007/s12011-015-0480-7.
31. Foroozanfard F, Jamilian M, Jafari Z, Khassaf A, Hosseini A, Khorammian H, et al. Effects of zinc supplementation on markers of insulin resistance and lipid profiles in women with polycystic ovary syndrome: a randomized, double-blind, placebo-controlled trial. Exp Clin Endocrinol Diabetes. 2015;123(4):215-20. doi:10.1055/s-0035-1548790.
32. Mohd Shukri MF, Norhayati MN, Badrin S, Abdul Kadir A. Effects of L-carnitine supplementation for women with polycystic ovary syndrome: a systematic review and meta-analysis. PeerJ. 2022;10:e13992. doi:10.7717/peerj.13992.
33. Samimi M, Jamilian M, Ebrahimi FA, Rahimi M, Tajbakhsh B, Asemi Z. Oral carnitine supplementation reduces body weight and insulin resistance in women with polycystic ovary syndrome: a randomized, double-blind, placebo-controlled trial. Clin Endocrinol (Oxf). 2016;84(6):851-7. doi:10.1111/cen.13003.
34. Melo V, Silva T, Silva T, Freitas J, Sacramento J, Vazquez M, et al. Omega-3 supplementation in the treatment of polycystic ovary syndrome (PCOS) - a review of clinical trials and cohort. Endocr Regul. 2022;56(1):66-79. doi:10.2478/enr-2022-0008.
35. Mirmasoumi G, Fazilati M, Foroozanfard F, Vahedpoor Z, Mahmoodi S, Taghizadeh M, et al. The Effects of Flaxseed Oil Omega-3 Fatty Acids Supplementation on Metabolic Status of Patients with Polycystic Ovary Syndrome: A Randomized, Double-Blind, Placebo-Controlled Trial. Exp Clin Endocrinol Diabetes. 2018;126(4):222-8. doi:10.1055/s-0043-119751.
36. Karakas SE, Perroud B, Kind T, Palazoglu M, Fiehn O. Changes in plasma metabolites and glucose homeostasis during omega-3 polyunsaturated fatty acid supplementation in women with polycystic ovary syndrome. BBA Clin. 2016;5:179-85. doi:10.1016/j.bbacli.2016.04.003.
37. Khani B, Mardanian F, Fesharaki SJ. Omega-3 supplementation effects on polycystic ovary syndrome symptoms and metabolic syndrome. J Res Med Sci. 2017;22:64. doi:10.4103/jrms.JRMS_644_16.
38. Izadi A, Shirazi S, Taghizadeh S, Gargari BP. Independent and Additive Effects of Coenzyme Q10 and Vitamin E on Cardiometabolic Outcomes and Visceral Adiposity in Women With Polycystic Ovary Syndrome. Arch Med Res. 2019;50(1):1-10. doi:10.1016/j.arcmed.2019.04.004.
39. Hariri Z, Yari Z, Hoseini S, Abhari K, Sohrab G. Synbiotic as an ameliorating factor in the health-related quality of life in women with polycystic ovary syndrome. A randomized, triple-blind, placebo-controlled trial. BMC Womens Health. 2024;24(1):19. doi:10.1186/s12905-023-02868-1.
40. Xia Y, Wang Y, Cui M, Su D. Efficacy of omega-3 fatty acid supplementation on cardiovascular risk factors in patients with polycystic ovary syndrome: a systematic review and meta-analysis. Ann Palliat Med. 2021;10(6):6425-37. doi:10.21037/apm-21-1050.
41. Yang K, Zeng L, Bao T, Ge J. Effectiveness of Omega-3 fatty acid for polycystic ovary syndrome: a systematic review and meta-analysis. Reprod Biol Endocrinol. 2018;16(1):27. doi:10.1186/s12958-018-0346-x.
42. Liu Z, Tian Z, Zhao D, Liang Y, Dai S, Liu M, et al. Effects of Coenzyme Q10 Supplementation on Lipid Profiles in Adults: A Meta-analysis of Randomized Controlled Trials. J Clin Endocrinol Metab. 2022;108(1):232-49. doi:10.1210/clinem/dgac585.
43. Raizner AE. Coenzyme Q10. Methodist Debakey Cardiovasc J. 2019;15(3):185-91. doi:10.14797/mdcj-15-3-185.
44. Laganà AS, Rossetti P, Buscema M, La Vignera S, Condorelli RA, Gullo G, et al. Metabolism and Ovarian Function in PCOS Women: A Therapeutic Approach with Inositols. Int J Endocrinol. 2016;2016:6306410. doi:10.1155/2016/6306410.
45. Facchinetti F, Orrù B, Grandi G, Unfer V. Short-term effects of metformin and myo-inositol in women with polycystic ovarian syndrome (PCOS): a meta-analysis of randomized clinical trials. Gynecol Endocrinol. 2019;35(3):198-206. doi:10.1080/09513590.2018.1540578.
46. Razavi M, Jamilian M, Karamali M, Bahmani F, Aghadavod E, Asemi Z. The Effects of Vitamin D-K-Calcium Co-Supplementation on Endocrine, Inflammation, and Oxidative Stress Biomarkers in Vitamin D-Deficient Women with Polycystic Ovary Syndrome: A Randomized, Double-Blind, Placebo-Controlled Trial. Horm Metab Res. 2016;48(6):446-51. doi:10.1055/s-0042-104060.
47. Karamali M, Ashrafi M, Razavi M, Jamilian M, Akbari M, Asemi Z. The Effects of Calcium, Vitamins D and K co-Supplementation on Markers of Insulin Metabolism and Lipid Profiles in Vitamin D-Deficient Women with Polycystic Ovary Syndrome. Exp Clin Endocrinol Diabetes. 2017;125(5):316-21. doi:10.1055/s-0043-104530.
48. Tarkesh F, Namavar Jahromi B, Hejazi N, Hoseini G. Effect of vitamin K2 administration on depression status in patients with polycystic ovary syndrome: a randomized clinical trial. BMC Womens Health. 2022;22(1):315. doi:10.1186/s12905-022-01825-8.
49. Huang Q, Liu Z, Yang Y, Yang Y, Huang T, Hong Y, et al. Selenium Nanodots (SENDs) as Antioxidants and Antioxidant-Prodrugs to Rescue Islet β Cells in Type 2 Diabetes Mellitus by Restoring Mitophagy and Alleviating Endoplasmic Reticulum Stress. Adv Sci (Weinh). 2023;10(13):e2300880. doi:10.1002/advs.202300880.
50. Li S, Zhao Q, Zhang K, Sun W, Li J, Guo X, et al. Selenium Deficiency-Induced Pancreatic Pathology Is Associated with Oxidative Stress and Energy Metabolism Disequilibrium. Biol Trace Elem Res. 2021;199(1):154-65. doi:10.1007/s12011-020-02140-9.
51. Di Lorenzo C, Ceschi A, Kupferschmidt H, Lüde S, De Souza Nascimento E, Dos Santos A, et al. The variability of nutritional supplement efficacy in clinical studies: A systematic overview. Nutrients. 2020;12(5):1350. doi:10.3390/nu12051350.
52. Vázquez-Lorente H, Molina-López J, Herrera-Quintana L, Gamarra-Morales Y, López-González B, Planells E. Confounding variables in clinical nutrition trials: An under-discussed issue. J Clin Med. 2021;10(1):88. doi:10.3390/jcm10010088.
53. Wang X, Zhang Y, Tan H, Zhao W, Zhao Y, Zheng W. Efficacy of monotherapy vs. co-supplementation in PCOS: A comparative analysis. Endocr Rev. 2019;40(3):517-33. doi:10.1210/er.2018-00238.
54. Ghanei L, Hariri M, Asbaghi O, Kashkooli S, Kashani A, Tavasoli S. Safety concerns and effectiveness of long-term vitamin D and selenium use in endocrine disorders. Clin Endocrinol (Oxf). 2022;97(6):851-60. doi:10.1111/cen.14801.
55. Hussain H, Al-Farhan H, Al-Otaibi S, Al-Massarani S, Al-Rasheed N. Melatonin supplementation in metabolic syndromes: Promises and limitations. Int J Endocrinol. 2023;2023:2548893. doi:10.1155/2023/2548893.
56. Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al. Cochrane Handbook for Systematic Reviews of Interventions. Version 6.3 (updated 2022). Cochrane; 2022.
57. Palomba S, Piltonen TT, Giudice LC. Nutraceutical approach to PCOS: clinical evidence and future perspectives. Hum Reprod Update. 2023;29(2):187-202. doi:10.1093/humupd/dmac032.
58. Vinceti M, Filippini T, Del Giovane C, Dennert G, Zwahlen M, Brinkman M, et al. Selenium for preventing cancer. Cochrane Database Syst Rev. 2018;1(1):CD005195. doi:10.1002/14651858.CD005195.pub4.
59. Fairweather-Tait SJ, Bao Y, Broadley MR, Collings R, Ford D, Hesketh JE, et al. Selenium in human health and disease. Antioxid Redox Signal. 2011;14(7):1337-83. doi:10.1089/ars.2010.3275.
60. Vieth R. Vitamin D toxicity, policy, and science. J Bone Miner Res. 2006;21(2):V64-V68. doi:10.1359/jbmr.06s223.
61. Ianiro G, Rossi E, Thomas AM, Schinzari G, Masucci L, Quaranta G, et al. Dietary supplements and drug interactions: a practical guide for clinicians. Eur Rev Med Pharmacol Sci. 2020;24(9):5095-100. doi:10.26355/eurrev_202005_21206.
62. Gurley BJ, Steelman SC, Thomas SL. Adulteration of dietary supplements and the need for improved regulatory oversight. Food Chem Toxicol. 2020;140:111346. doi:10.1016/j.fct.2020.111346.
63. Goodarzi MO, Dumesic DA, Chazenbalk G, Azziz R. Genetics of PCOS and its implication for diagnosis and treatment. Best Pract Res Clin Endocrinol Metab. 2011;25(2):139-52. doi:10.1016/j.beem.2010.08.015.
64. Cohen PA. American roulette — contaminated dietary supplements. N Engl J Med. 2014;371(18):1763-6. doi:10.1056/NEJMp1405831.
65. Jamilian M, Foroozanfard F, Mirhosseini N, Bahmani F, Taghizadeh M, Asemi Z. Effects of melatonin supplementation on hormonal, inflammatory, genetic, and oxidative stress parameters in women with polycystic ovary syndrome. Front Endocrinol (Lausanne). 2019;10:273. doi:10.3389/fendo.2019.00273
Received: December 7, 2024 / Accepted: April 17, 2025 / Published: June 15, 2025
Citation: Andres-Montenegro N, Molina-Gutierrez S, Núñez-Murillo O. Evidence-Based Nutritional Supplementation in Polycystic Ovary Syndrome: A Comprehensive Review of Clinical Outcomes, Mechanisms, and Recommendations. Bionatura journal. 2025;2 (2):13. doi: 10.70099/BJ/2025.02.02.13
Additional information Correspondence should be addressed to olinda.nunez@unah.edu.hn
Peer review information. Bionatura thanks anonymous reviewer(s) for their contribution to the peer review of this work using https://reviewerlocator.webofscience.com/
ISSN.3020-7886
All articles published by Bionatura Journal are made freely and permanently accessible online immediately upon publication, without subscription charges or registration barriers.
Publisher's Note: Bionatura Journal stays neutral concerning jurisdictional claims in published maps and institutional affiliations.
Copyright: © 2025 by the authors. They were submitted for possible open-access publication under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).


