Terminological Rigor in Nanomedicine: Standardizing 'Nano' Descriptors for Reproducibility and Translational Success
ABSTRACT
The rapid growth of nanomedicine has revolutionized drug delivery innovations. Nevertheless, the scientific credibility is jeopardized by the uncritical use of "nano" terminology, which is frequently lacking in robust physicochemical or functional validation. This editorial denounces the misuse of terms such as "targeted delivery," "smart carriers," and "nanoparticles," which are frequently employed without experimental justification. We recommend concrete measures to standardize terminology, encouraging authors, journal editors, and educators to implement evidence-based definitions. To guarantee reproducibility, regulatory transparency, and successful clinical translation in nanomedicine, it is imperative to achieve rigorous semantic precision.
Keywords: nanomedicine, terminology standardization, drug delivery systems, scientific rigor, nanocarriers, size characterization, stimuli-responsive systems, targeted delivery, regulatory reproducibility, translational nanotechnology, EPR effect, nanoparticle characterization, academic integrity
INTRODUCTION
The misuse of "nano" terminology in drug delivery research risks misleading clinicians, regulators, and funding agencies, thereby impeding translational progress. This editorial pinpoints critical deficiencies in semantic rigor and suggests practical solutions.
The guarantee of clarity and precision in scientific terminology is a fundamental component of academic integrity and progress as nanomedicine continues to develop as a prominent area of pharmaceutical research. This editorial examines the deteriorating precision of the term "nano" in the field of drug delivery literature and calls for a return to rigor and discipline in nomenclature. The field of drug delivery has undergone a significant surge in innovation in recent years, which has been categorized under the term "nanomedicine"¹. Although this expansion is commendable, there is an increasing apprehension regarding the diluted nature of the term "nano," which is frequently employed as a marketing tool rather than a precise scientific classification.
This concern echoes findings by Wilhelm et al., who demonstrated that the actual tumor delivery of nanoparticles remains below 1%, highlighting the need for honest characterization and reporting².
TERMINOLOGY IN SIZE AND FUNCTION
Nanosystems are typically defined as particles with a size between 1 and 100 nm, or ones that have at least one dimension below 10 nm³. Nevertheless, a growing number of publications refer to formulations as "nanocarriers" or "nanosystems" without providing a clear justification based on structural, functional, or size attributes. The term "nanoparticles" or "nanoclusters" is still used to describe systems with hydrodynamic diameters that exceed 300 nm, which are well outside the optimal range for passive tumor targeting via the enhanced permeability and retention (EPR) effect⁴.
Rodríguez et al. have previously demonstrated that even polymeric nanocarriers with well-defined physicochemical profiles can exhibit unpredictable biodistribution without precise size control and functional validation⁵.
In some cases, particles approaching or exceeding the micron scale are similarly labeled, potentially misleading readers about their biological behavior and implications for in vivo application⁶.
The same imprecision applies to functional claims. Terms such as "targeted," "smart," or "stimuli-responsive" are frequently employed without adequate mechanistic evidence. For example, a delivery system may be labeled as "targeted" solely because of a surface ligand, without proof of receptor-specific uptake or enhanced therapeutic indices compared to non-targeted controls. Similarly, claims of stimulus responsiveness frequently lack kinetic data under physiologically relevant conditions⁷.
Industry-focused reviews have flagged this gap between mechanistic claims and experimental validation as a major barrier to clinical translation².
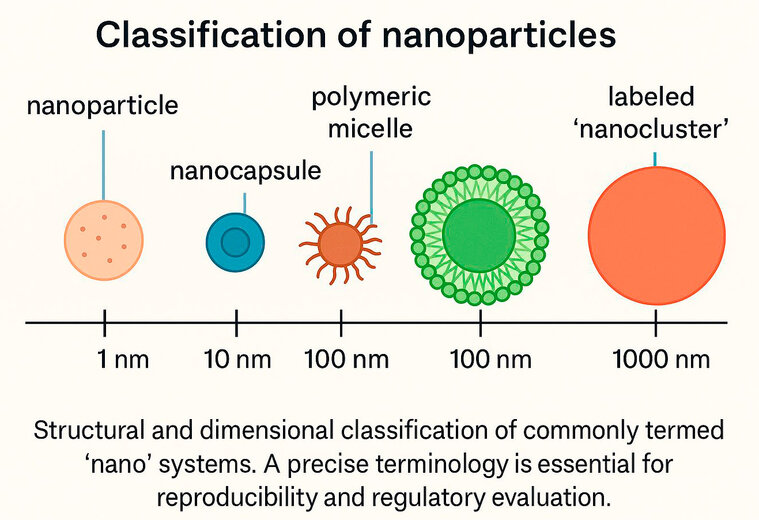
Figure 1. Size-based classification of nanocarriers: Mislabeling particles beyond 100 nm as "nano"—such as 300 nm systems—distorts their biological interpretation, particularly regarding EPR effect, cellular uptake, and biodistribution. Accurate size characterization using TEM, DLS, and polydispersity index (PDI) is mandatory for evidence-based classification.
DISCUSSION AND RECOMMENDATIONS
Terminological inflation of this kind not only undermines scientific rigor but may also diminish the trust of clinicians, regulators, and funding agencies in the promises of nanotechnology. The repeated failure of "smart nanocarriers" to deliver meaningful translational benefits calls for greater responsibility in describing and validating these systems.
Rodríguez and Sánchez have emphasized that lipid-based nanocarriers must be thoroughly validated in physiologically relevant models to support their claimed advantages in cancer therapy⁸.
Therefore, authors must employ "nano" descriptors only when supported by robust physicochemical characterization techniques—such as DLS, TEM, and polydispersity index—and reliable experimental data substantiates that functional claims like targeting or responsiveness. Comparative controls, including conventional (non-nano) systems, should be incorporated to contextualize any claimed enhancements in performance.
The broader research community benefits from a shared commitment to terminological precision. This would facilitate reproducibility and scientific dialogue and enhance regulatory and clinical translation. Journals might consider adopting editorial policies or checklists to guide authors' terminology and claims, aligning submissions with best practices in the field.
Such frameworks have been advocated in regulatory discussions on nanomedicine standardization and quality assurance².
Educators also have a vital role to play. Graduate programs in pharmaceutical sciences, biomedical engineering, and related disciplines should emphasize critical thinking about language and evidence in research. Instilling these values early will help cultivate a generation of scientists capable of advancing nanomedicine with innovation and integrity.
CONCLUSIONS
As the field matures, its credibility will increasingly depend on our commitment to clarity and methodological rigor. In scientific publishing, precision in language is more than semantics—it reflects our responsibility to the truth.
The precise utilisation of "nano" terminology in drug delivery research is not a matter of semantics, but a fundamental component of scientific rigor, reproducibility, and translational success. Misused size-based and functional descriptors may result in misconceptions and undermine credibility in academic and regulatory contexts. Moving forward, authors must provide a solid foundation for their use of the term "nano" by demonstrating validated functionality and robust characterization.
We recommend that journals implement mandatory checklists for nano-related terminology, encompassing size thresholds, experimental validation, and control comparisons. Funding agencies should prioritize projects that demonstrate terminological precision, and these standards must be incorporated into scientific training programs. A common commitment to methodological and linguistic accuracy will strengthen the field of nanomedicine's base and hasten its transition to useful clinical applications.
Funding
This research received no external funding.
Acknowledgments
The authors would like to thank the Yachay Tech community for their support. No specific grants from public, commercial, or not-for-profit funding agencies were used for this work.
Conflicts of Interest
The authors declare no conflict of interest.
REFERENCES
- Park K. Facing the Truth about Nanotechnology in Drug Delivery. ACS Nano. 2013;7:7442–7447. doi:10.1021/NN404501G
- Wilhelm S, Tavares AJ, Dai Q, et al. Analysis of nanoparticle delivery to tumours. Nat Rev Mater. 2016;1(5):16014. doi:10.1038/natrevmats.2016.14
- Öztürk K, Kaplan M, Çalış S. Effects of Nanoparticle Size, Shape, and Zeta Potential on Drug Delivery. Int J Pharm. 2024;666:124799. doi:10.1016/J.IJPHARM.2024.124799
- Xia S, Duan Y, Yu S, et al. A Cellulosic Multi-Bands Fluorescence Probe for Rapid Detection of PH and Glutathione. Carbohydr Polym. 2024;331:121893. doi:10.1016/J.CARBPOL.2024.121893
- Ramos TI, Villacis-Aguirre CA, Sandoval FS, Martin-Solano S, Manrique-Suárez V, Rodríguez H, Santiago-Padilla L, Debut A, Gómez-Gaete C, Arias MT, et al. Multilayer Nanocarrier for the Codelivery of Interferons: A Promising Strategy for Biocompatible and Long-Acting Antiviral Treatment. Pharmaceutics. 2024; 16(11):1349. https://doi.org/10.3390/pharmaceutics16111349
- Talele P, Jadhav A, Sahu S, Shimpi N. Experimental Approaches to Evaluate Solid Lipid Nanoparticle-Based Drug Delivery Systems. Anal Methods. 2025;17:1451–1466. doi:10.1039/D4AY01659A
- Shreyash N, Sonker M, Bajpai S, Tiwary SK. Review of the Mechanism of Nanocarriers and Technological Developments in the Field of Nanoparticles for Applications in Cancer Theragnostics. ACS Appl Bio Mater. 2021;4:2307–2334. doi:10.1021/ACSABM.1C00020
- Ruiz, A.;
Suárez, M.; Martin, N.; Albericio, F.;
Rodríguez, H. Beilstein J. Nanotechnol. 2014, 5, 374–379. doi:10.3762/bjnano.5.43
Citation: Rodriguez H. Terminological Rigor in Nanomedicine: Standardizing 'Nano' Descriptors for Reproducibility and Translational Success. Bionatura Journal. 2025;2 (2):1. doi: 10.70099/BJ/2025.02.021.1
Additional information: Correspondence should be addressed to hmrodriguez@yachaytech.edu.ec
Peer review information. Bionatura thanks anonymous reviewer(s) for their contribution to the peer review of this work using https://reviewerlocator.webofscience.com/
ISSN.3020-7886
All articles published by Bionatura Journal are made freely and permanently accessible online immediately upon publication, without subscription charges or registration barriers.
Publisher's Note: Bionatura Journal stays neutral concerning jurisdictional claims in published maps and institutional affiliations.
Copyright: © 2025 by the authors. They were submitted for possible open-access publication under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).